Pipeline
INFLUENZA IN IMMUNOCOMPROMISED PATIENTS
In immunocompromized patients, Influenza is associated with a significantly higher number of radiographic abnormalities and severe complications and, requires more intensive care and mechanical ventilation.
Prolonged influenza shedding is also observed in these patients which contributes to viral spread in the general population.
- The population with the highest risk of mortality are haematopoietic stem cell transplant (HSCT) patients, but also other conditions such as:
– Hematologic malignancies and solid organ tumor patients
– Patients who receive other immunosuppressive therapies
- In these patients Influenza vaccines have highly variable efficacy that is hard to predict
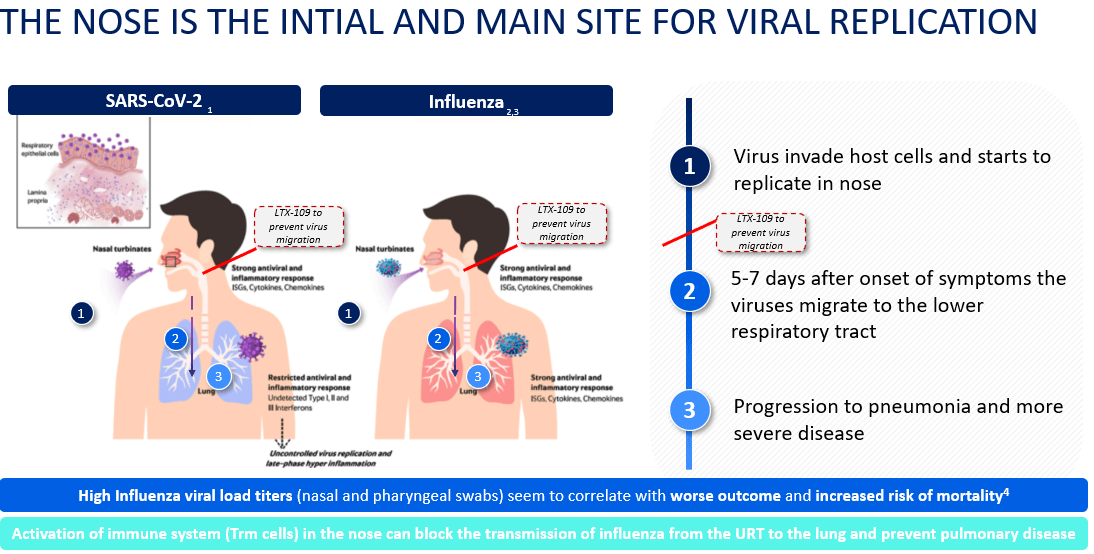
- High Influenza viral load titers seem to correlate with worse outcome and increased risk of mortality
- Current drugs do not have a meaningful impact on symptom duration in these patients, and they are replication inhibitors in contrast to LTX-109s direct virucidal effect.
LTX-109, as a direct virucidal, will demonstrate reduction in hospitalisation, mortality and symptom duration in clinical trials in non-hospitalized early stage HSCT influenza patients as an add-on to standard of care.
Anti-bacterial work
NASAL DECOLONISATION
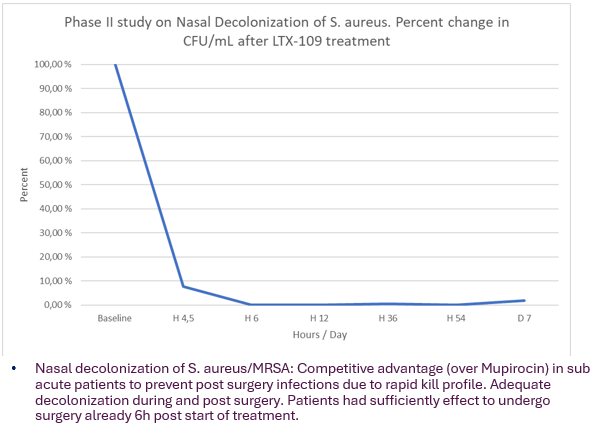
- Nasal decolonization is a preventive measure to reduce risk of post-surgery infection in high-risk surgery and is recommended by WHO.
- Mupirocin is currently the ‘gold standard’ drug for Nasal decolonization. However, patients need to be treated for 5 days prior to surgery for Mupirocin to be effective.
- Mupirocin is therefore not suitable for sub-acute patients (undergoing surgery within 12- 24h).
- LTX-109 gel formulation aims to bridge this gap with its rapid acting profiles.
